

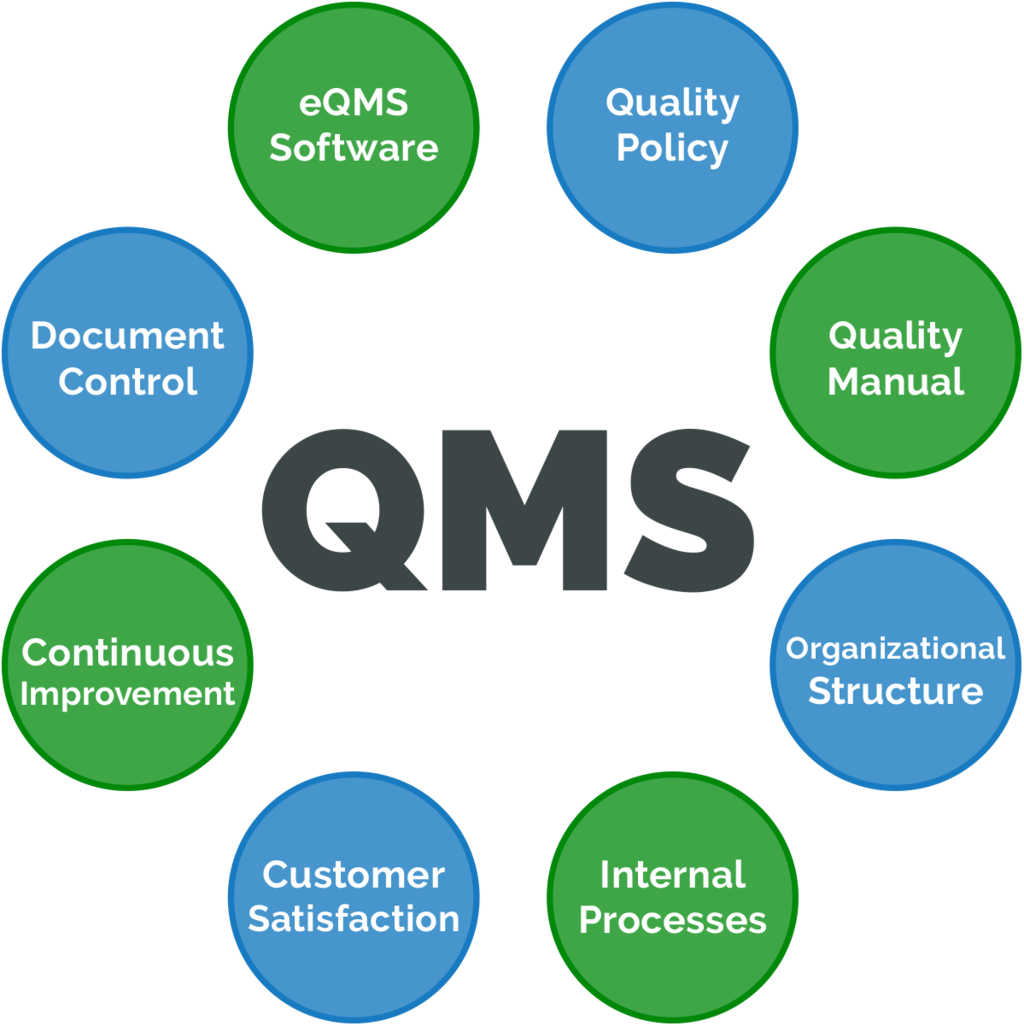
Consultation and Implementation: Support companies in designing, implementing, and maintaining a robust Quality Management System (QMS) aligned with ISO 13485 or other relevant standards.
Training and Audits: Offer training sessions for staff on QMS principles and perform internal audits to ensure compliance.
Risk-Based Approach: Assist organizations in integrating risk management practices into their QMS.


Projects done
Happy Clients
Years of Experience
People Working